全球首創簡易尿液HPV檢測,測試靈敏度超越柏氏抹片,能檢測出14種可致癌高風險HPV 病毒。只需採集尿液樣本,便可輕鬆進行,無需痛苦的拭子採樣。準確度高,私密性強!
Patented Technology for Urine DNA Concentration

- 採用 PHASiFY™ 技術處理大量尿液樣本,超濃縮及純化樣本中的游離基因
- Enhance the sensitivity and accuracy of urine samples
- Avoid embarrassment through self-collection of urine sample
- Allow you to analyze your cervical cancer risk early
Understanding cervical cancer,
learning about HPV testing
WHO recommends that HPV test is the most cost-effective technique for cervical screening. Detection of HPV infections that leads to cellular transformation followed by timely treatment can lower the risk of developing over 95% of cervical cancer1。
Starting from 2023, the Department of Health of Hong Kong also enhanced its cervical cancer screening services by replacing Pap smear tests with a higher sensitivity HPV test4.
HPV infection can be easily transmitted through any intimate skin-to-skin contact, incl. vaginal–penile sex, penile–anal sex, penile–oral sex, vaginal–oral sex, and use of sex toys or other objects5.
感染高風險 HPV 及其引致的癌前病變甚少會引起明顯的症狀,女性一般會於持續感染後 15-20 年才會發展成子宮頸癌。因此,進行恆常 HPV 檢測作為子宮頸癌篩查尢其重要。
一些低風險 HPV 病毒屬於可能致癌的 2A 及 2B 類致癌物6; while some may cause genital warts on the prepuce, glans of the penis, urethral opening, anus, vulva, labia, vagina or cervix7.
HPV vaccine is effective in preventing the targeted HPV types that most commonly cause cervical cancer and genital warts, but not all types of HPV that can cause cervical cancer8.
Also, HPV vaccine does not treat existing HPV infections, which is why regular HPV screening tests for cervical cancer are strongly recommended8.
80% of men & women will acquire HPV in their lifetime9


Everyone should get tested, especially high-risk individuals11
All-rounded support
Put your health in your hands
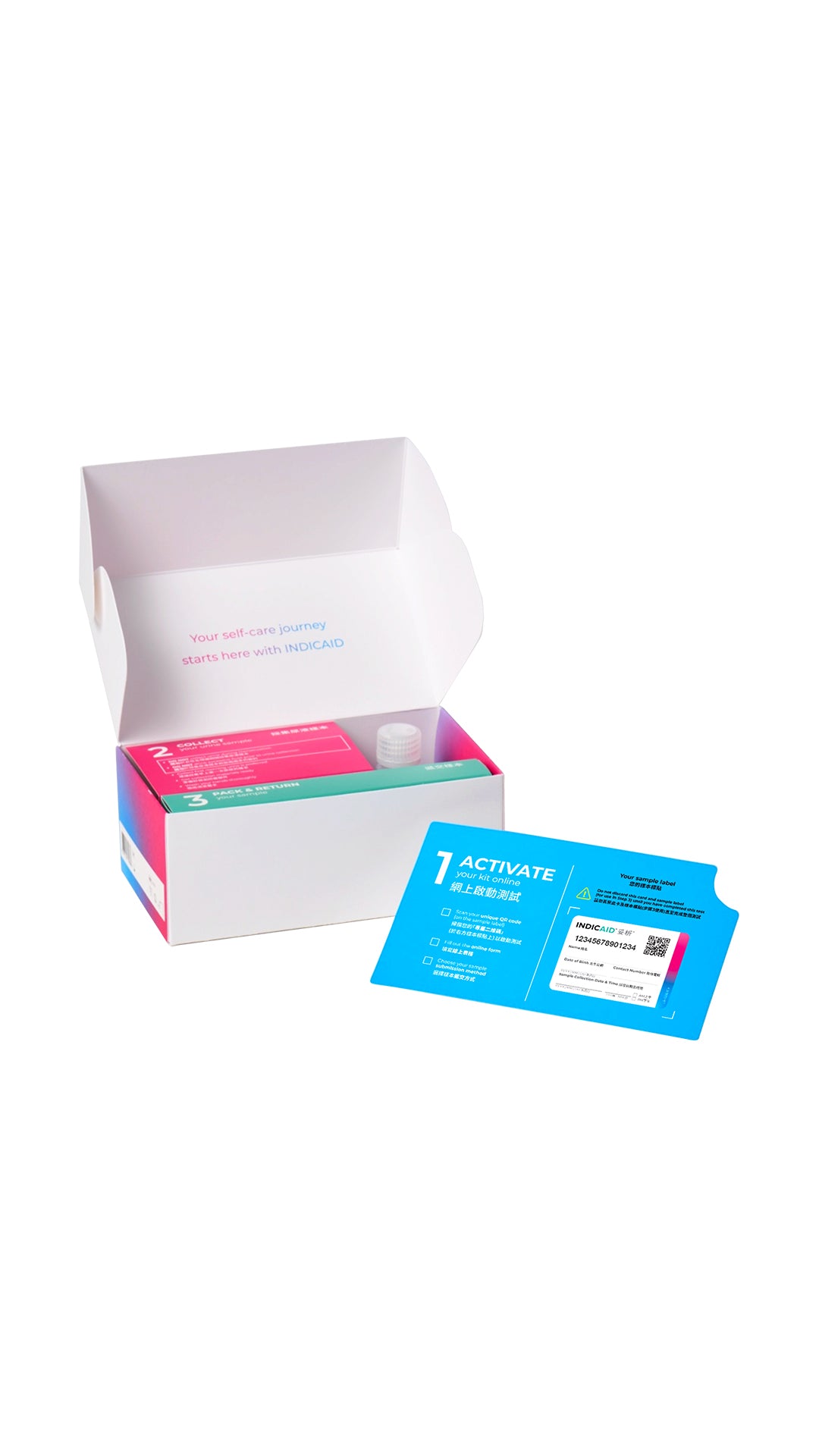
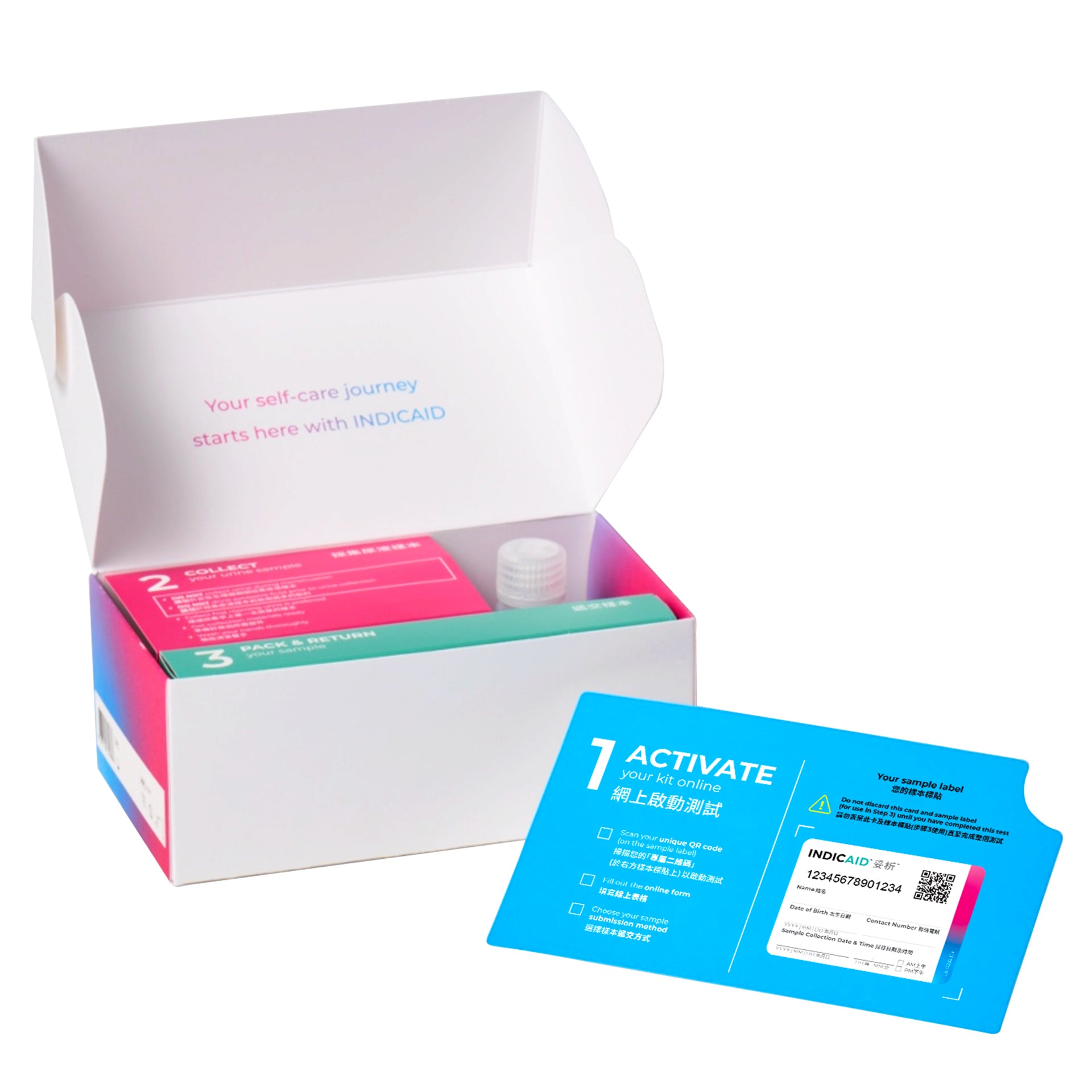
1. Urine Self-Test Kit
- Urine collection bottle
- Preservative
- Sample bag
- Outer bag
- Sample label
- Disposable gloves (1 pair)
2. Digital Lab Test Report
Digital lab test report issued via SMS and email 5 working days after the lab receives the sample.
3. Follow-up services for positive HPV test result
如檢測HPV病毒陽性,我們會主動與你聯絡提供協助。
Detailed instructions, simple and easy to use
Activate your kit online*
Collect your urine sample
Free Sample pick-up/drop-off service
How to return your sample?
Free Pick-up
Before Your Sample Submission
- 掃瞄您的二維碼,填妥線上表格和選擇樣本遞交方式
- 已將保存液倒入已載有尿液的採樣瓶中
- 已填寫樣本標貼並張貼在採樣瓶上
- Had your sample picked up / dropped off on or before the expiry date listed on the test kit
More About Sample Pick-up
Pick-up service is currently unavailable for remote areas. You can choose to drop off your sample to our drop-off points.
What's next
Different follow-up actions should be made depending on the HPV results.


Detected
Please seek medical advice. More regular HPV screening tests are recommended for continuous monitoring.
如檢測HPV 病毒陽性,我們會主動與您聯絡提供協助。
Not Detected
Routine HPV screening tests for cervical cancer should be done. If you experience any swelling, bleeding, pain or other discomfort, seek medical advice promptly.
Earlier analysis of your cervical cancer risk
Highly Recommended by Users
一路都好怕做HPV病毒檢測或者子宮頸抹片檢查,又尷尬又痛。而家用 INDICAID自己喺屋企採集尿液就可以驗到27種HPV。
Karen Cheng
愛錫自己,梗係要每年做一次身體檢查啦!INDICAID HPV 子宮頸癌篩查測試係真正嘅 home test,唔使再怕尷尬,輕鬆無痛就可以發現 HPV 同癌症風險。
陳嘉倩
對上一次做抹片檢查係疫情前,已經4至5年前。要打電話預約好麻煩,時間又難夾,返工要請假亦唔容易,而家自己做HPV測試可以慳返好多時間!
賴女士
上年家姐中咗HPV病毒,係九價疫苗以外嘅HPV,自己先開始留意。發現去醫院做檢查都幾尷尬幾麻煩。有INDICAID就可以自己搞掂唔使煩啦!
肖女士
結婚前同生小朋友前我都出去做過2次柏氏抺片,原來依家有更輕鬆簡單的方法,無痛、無尷尬、自我尿液採樣驗 HPV,我喜歡呢個方法!
Mama Amiko
一直知道驗HPV 好重要,但成日揍住小朋友吾得閒親身去做檢查。而家可以足不出戶, 在家中尿液採樣檢, 再遞交樣本去化驗所就可以。
Mama Chanbo
宮頸抺片檢測非常唔舒服,好多姊妹都因此唔去年年驗身。不過而家可以無痛,自我採樣,留小便驗HPV,好方便
Cman
Explore more
FAQs
INDICAID™妥析™ HPV 尿液樣本測試與柏氏抺片檢查屬於兩種不同的技術。
INDICAID™妥析™ HPV 尿液樣本測試主要檢測用家的尿液樣本中是否存有 HPV 病毒;而柏氏抺片則是由化驗所人員透過顯微鏡,檢視子宮頸細胞樣本有否出現異常,判斷子宮頸是否存在癌前病變。
If your HPV DNA test detects HPV from your sample, you may consider undergoing a Pap smear test to verify if there is any abnormalities with your cervical cells.
It is suitable for both men and women. It is reported that 80% of men and women will acquire HPV in their lifetime, everyone can transmit HPV to their partners. Therefore, it is recommended to get tested for HPV regularly to protect yourself and your partner9。
部分市場上的 HPV 測試產品要求自行於陰道內採樣,因此只適合女士使用。INDICAID™妥析™ HPV 尿液樣本測試是透過尿液樣本來檢測用家是否存有 HPV DNA,因此沒有性別限制,男女同時適用。
Activate your kit online
- 掃描您的專屬二維碼
- 填寫線上表格
- Choose your sample submission method
- 採集排尿的首段 90-100亳升尿液樣本於採樣瓶內
- 將保存液倒入已載有尿液的採樣瓶中
- Re-cap tightly the filled urine collection bottle. Gently invert 3-5 times to mix the urine with the preservative
- 填寫樣本標貼並張貼在已裝滿尿液的採樣瓶上
- 將採樣瓶放入樣本袋中並將其密封
- 將封好的樣本袋放入外包裝袋中並將其密封
- 必須於採樣後24小時內根據已選擇的樣本遞交方式遞交樣本
電子化驗報告將於樣本送達化驗所後5個工作天內以手機短訊及電郵發出
Please contact our customer service at 3700 8888 for a receipt.
References
- Bruni L, Albero G, Serrano B, Mena M, Collado JJ, Gómez D, Muñoz J, Bosch FX, de Sanjosé S. CO/IARC Information Centre on HPV and Cancer (HPV Information Centre). Human Papillomavirus and Related Diseases in the World. Summary Report 10 March 2023.
- Marx, V. Method of the year: long-read sequencing. Nat Methods 20, 6–11 (2023).
- Centre for Health Protection: Health Topics on Cervical Cancer (2023).
- Department of Health: DH introduces human papillomavirus testing and calls on public to undergo regular cervical cancer screening (2023).
- Okunade KS. Human papillomavirus and cervical cancer. J Obstet Gynaecol. 2020 Jul;40(5):602-608. doi: 10.1080/01443615.2019.1634030. Epub 2019 Sep 10. Erratum in: J Obstet Gynaecol. 2020 May;40(4):590. PMID: 31500479; PMCID: PMC7062568.
- International Agency for Research on Cancer: IARC Monographs on the Identification of Carcinogenic Hazards to Humans (2023).
- The Family Planning Association of Hong Kong: Health Info on Sexually Transmissible Diseases. Accessed on 30th June 2023.
- Chesson, Harrell W. PhD; Dunne, Eileen F. MD, MPH; Hariri, Susan PhD; Markowitz, Lauri E. MD. The Estimated Lifetime Probability of Acquiring Human Papillomavirus in the United States. Sexually Transmitted Diseases 41(11):p 660-664, November 2014.
- Scientific Committee on Vaccine Preventable Diseases and Scientific Committee on AIDS and STI, Centre for Health Protection: Consensus Statement on the use of Human Papillomavirus (HPV) Vaccine in prevention of cervical cancer (2016).
- Cancer Expert Working Group on Cancer Prevention and Screening (CEWG), Centre for Health Protection: Recommendations on Prevention and Screening for Cervical Cancer For Health Professionals (2021).








